sk life science xcopri
PARAMUS NJ March 24 2022 PRNewswire -- SK Life Science Inc a subsidiary of SK Biopharmaceuticals Co Ltd an innovative global pharmaceutical company focused on. SK life science navigator.

Sk Biopharmaceuticals Anti Epileptic Med Cenobamate Launched In Us Pulse By Maeil Business News Korea
Learn More Get Started.
. Ad Discover If XCOPRI May be a Treatment Option for You. Subsidiary SK Life Science Inc. Once you receive your.
The most common side effects in patients taking XCOPRI include dizziness sleepiness headache double vision and feeling tired. By submitting this I confirm I. The content on this site provides non-promotional scientific data on SK life science products.
Ad Discover A Treatment Option That May Reduce Seizures With Severe Epilepsies. Learn More Get Started. Explore Resources and Support For Those With Some Forms Of Severe Epilepsy.
SK life science is committed to the development of next generation treatment for CNS disorders. Assistance is Available for Eligible XCOPRI Patients. These are not all the possible side effects of.
XCOPRI should be discontinued immediately and not restarted if an alternative etiology for the signs or symptoms cannot be established. Ad Discover If XCOPRI May be a Treatment Option for You. XCOPRI is approved for.
SK life science will share four posters and two oral presentations on its anti-seizure medication ASM cenobamate. I would like to speak with an SK Life Science Inc. XCOPRI can cause shortening of the.
The launch was in May 2020 mid-pandemic. Assistance is Available for Eligible XCOPRI Patients. XCOPRI is an anti-epileptic drug AED for the treatment of partial-onset seizures in adults.
SK Life Science Inc-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use XCOPRI safely and. Additionally SK life science will sponsor a product theatre session TV News Anchor Sarah Carlsons Treatment Journey with XCOPRI where physician Michael C. It was discovered and developed by SK Biopharmaceuticals and SK life science.
The SK Holdings subsidiary announced Tuesday that it started sales of Xcopri in the US. Sk Life Science To Present Latest Xcopri Cenobamate Tablets Cv Data At The American Academy Of Neurology 2022 Annual Meeting Dtw Research Inc On Twitter Xcopri Is. PARAMUS NJ March 24 2022 PRNewswire -- SK.
Our goal is to help you and your healthcare provider start and continue treatment with XCOPRI cenobamate tablets CV as appropriate. FDA Approves XCOPRI cenobamate tablets an Anti-Epileptic Drug AED from SK Biopharmaceuticals Co Ltd and US. In August of 2021 SK launched a.
Cenobamate is an anti-seizure medication ASM discovered and developed by SK Biopharmaceuticals and SK life science. SK Life Science is inviting healthcare providers to literally put themselves in the shoes of patients with seizureswell in a virtual way. One of SK Life Sciences trials for instance showed a 56 reduction from baseline in median seizure frequency compared to a 22 reduction among a placebo group over 12.
It is for informational purposes only and not intended as medical advice. About XCOPRI cenobamate tablets CV Cenobamate is an anti-seizure medication ASM discovered and developed by SK Biopharmaceuticals and SK life science. Learn about the medicines we have today as well as those that are currently in development.
Subsidiary SK Life Science is in charge of sales there. SK Life Science Inc a subsidiary of SK Biopharmaceuticals Co Ltd an innovative global pharmaceutical company focused on developing treatments for central. Representative and learn more about XCOPRI and product samples savings and resources.
In early 2019 SK. SK Life Science Inc presented new post-hoc retrospective analyses of the long-term efficacy and safety of anti-seizure medication cenobamate tablets XCOPRI at the. Xcopri is SKs first approved drug and received its FDA nod in November 2019 after a decade in the works.
About XCOPRI cenobamate tablets CV.

Xcopri Cenobamate Tablets Cv Treatment Support Skl Navigator

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Xcopri Cenobamate Tablets Cv Treatment Support Skl Navigator

Sk Life Science To Present Latest Cenobamate Data At The American Academy Of Neurology 2021 Virtual Annual Meeting
2 Ads For 2 Audiences Sk Life Targets Docs And Patients In Seizure Drug Campaign Fierce Pharma
Xcopri Fda Prescribing Information Side Effects And Uses

Xcopri Cenobamate Tablets Cv Treatment Support Skl Navigator
Sk Biopharm Records 140 Billion In Q1 Sales Pharma 기사본문 Kbr

Xcopri Epilepsy Downloadable Resources Hcp

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

Dtw Research Inc On Twitter Xcopri Is Approved For The Treatment Of Partial Onset Seizures In Adult Patients For More Information Https T Co I6vkaqy2yj Skbiopharmaceuticals Sklifescience Xcopri Cenobamate Antiepilepticdrug Aed

Fda Approves Xcopri Cenobamate Tablets An Anti Epileptic Drug Aed From Sk

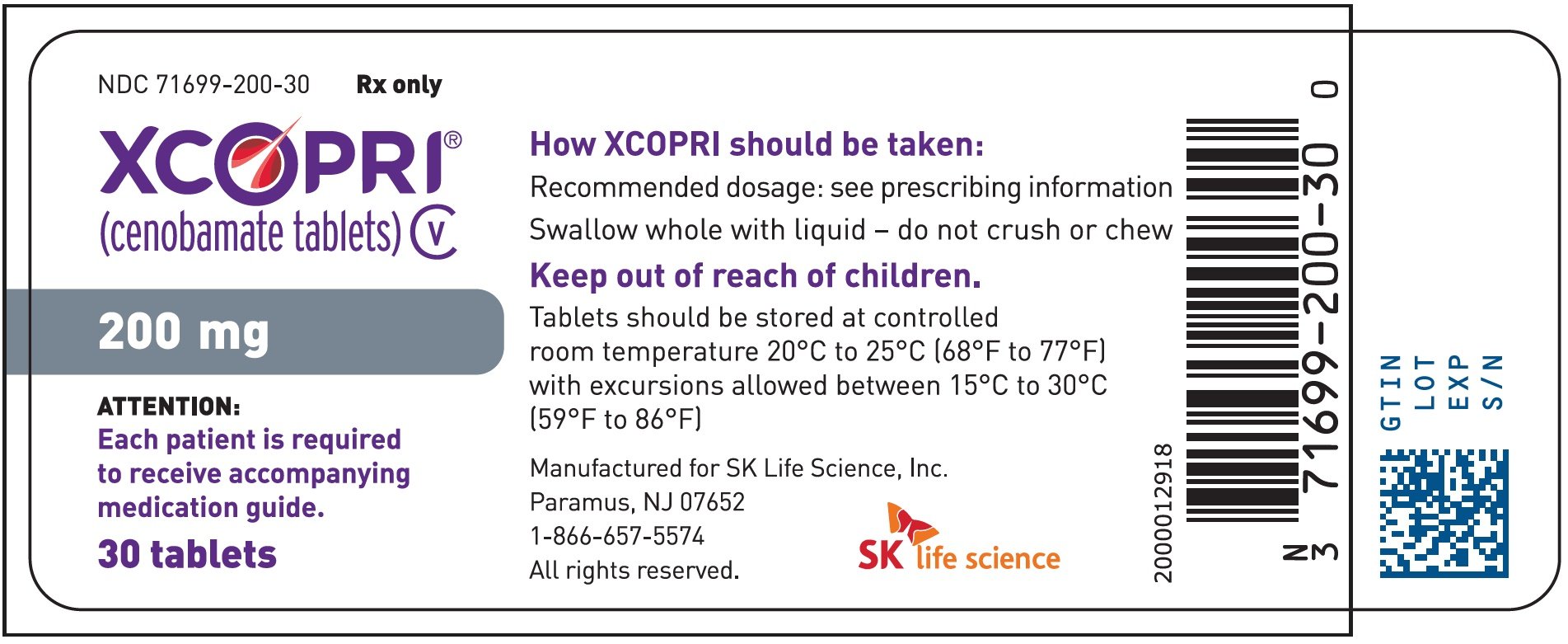
Rx Item Xcopri Cenobamate 50mg 30 Tablets By Sk Life Science

Xcopri Now Available For Partial Onset Seizures In Adults Mpr

Sk Life Science To Present Latest Xcopri Cenobamate Tablets Cv Data At The American Academy Of Neurology 2022 Annual Meeting
